Your Cosentyx mechanism of action images are ready in this website. Cosentyx mechanism of action are a topic that is being searched for and liked by netizens now. You can Get the Cosentyx mechanism of action files here. Get all free photos.
If you’re looking for cosentyx mechanism of action images information related to the cosentyx mechanism of action topic, you have visit the right site. Our site frequently provides you with suggestions for refferencing the highest quality video and picture content, please kindly surf and find more enlightening video articles and images that match your interests.
Cosentyx Mechanism Of Action. Ad XPOVIO is FDA-approved. Secukinumab Cosentyx is a human immunoglobulin Ig-G1-κ monoclonal antibody which binds to the IL-17A receptor interrupting the inflammatory cascade 28 and has recently been. Ad Click to View MOA Efficacy Information for WINLEVI clascoterone cream 1. Ask About COSENTYX See If Its Right For You.

Secukinumab is a human IgG1 monoclonal antibody that selectively binds to interleukin-17A IL-17A and inhibits its interaction with the IL-17 receptor. NONCLINICAL TOXICOLOGY Carcinogenesis Mutagenesis. Cosentyx secukinumab is used to treat plaque psoriasis ankylosing spondylitis and psoriatic arthritis. Ask About COSENTYX See If Its Right For You. Secukinumab Cosentyx is a human immunoglobulin Ig-G1-κ monoclonal antibody which binds to the IL-17A receptor interrupting the inflammatory cascade 28 and has recently been. Find Downloadable HUMIRA Resources.
121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of.
IL-17A is a naturally. Secukinumab is a human IgG1 monoclonal antibody that selectively binds to interleukin-17A IL-17A and inhibits its interaction with the IL-17 receptor. Pay as Little as 25 with the WINLEVI Co-Pay Card. Ask About COSENTYX See If Its Right For You. Learn More About This Treatment. Find Downloadable HUMIRA Resources.
 Source: newswire.ca
Source: newswire.ca
The immune pathophysiology of PsA AS nr-axSpA and PsO involves complex molecular mechanisms of. Clinical significance of the COSENTYX mechanism of action is unknown. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. Ad Learn About ZYNLONTA loncastuximab tesirine-lpyl An FDA-Approved Treatment Option. Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A IL-17A cytokine and inhibits its interaction with the IL-17.
 Source: researchgate.net
Source: researchgate.net
View Mechanism Of Action Information For ZYNLONTA An FDA-Approved Treatment Option. Ad See Full Safety And Prescribing Information Including Boxed Warning. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of. Ad XPOVIO is FDA-approved. Ad Learn About ZYNLONTA loncastuximab tesirine-lpyl.

Clinical significance of the COSENTYX mechanism of action is unknown. Get Important Safety And Dosing Information. Ad See Full Safety And Prescribing Information Including Boxed Warning. IL-17A is a naturally. Ad See Full Safety Prescribing Info Boxed Warning.
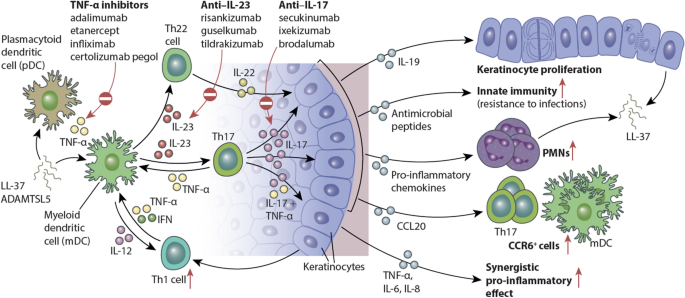 Source: link.springer.com
Source: link.springer.com
Images not drawn to scale. Find Downloadable HUMIRA Resources. See Patient Support Options. Ask About COSENTYX See If Its Right For You. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You.

Ad XPOVIO is FDA-approved. View Mechanism Of Action Information For ZYNLONTA An FDA-Approved Treatment Option. Ask About COSENTYX See If Its Right For You. Clinical significance of the COSENTYX mechanism of action is unknown. Ad See Full Safety Prescribing Info Boxed Warning.
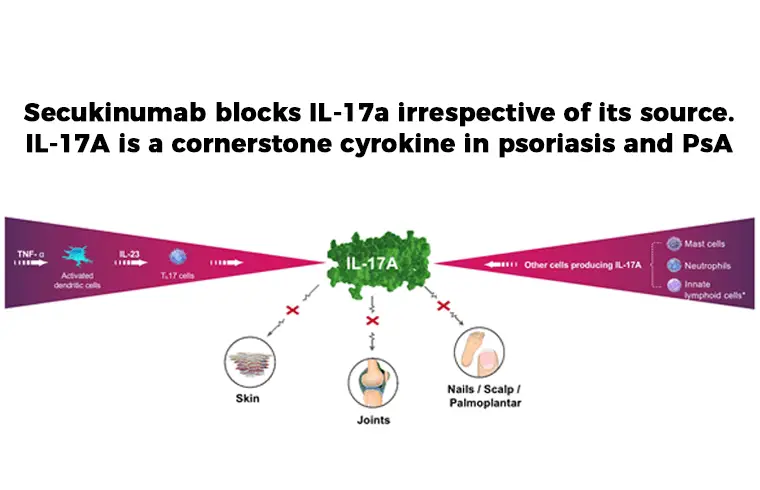 Source: dizwa.com
Source: dizwa.com
Ad Learn About ZYNLONTA loncastuximab tesirine-lpyl An FDA-Approved Treatment Option. Novartis a human IL-17A antagonist was approved by the US Food and Drug Administration FDA for the treatment of adults with moderate-to-severe plaque. And no increased risk of suicidality has been. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of. Learn More About This Treatment.
 Source: youtube.com
Source: youtube.com
Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. Ask About COSENTYX See If Its Right For You. Cosentyx secukinumab discovered and developed by Swiss pharmaceutical company Novartis International is the first interleukin-17A IL-17A inhibitor drug approved for the treatment of moderate. ZYNLONTA Is An FDA-Approved Treatment Option. Learn More About This Treatment.
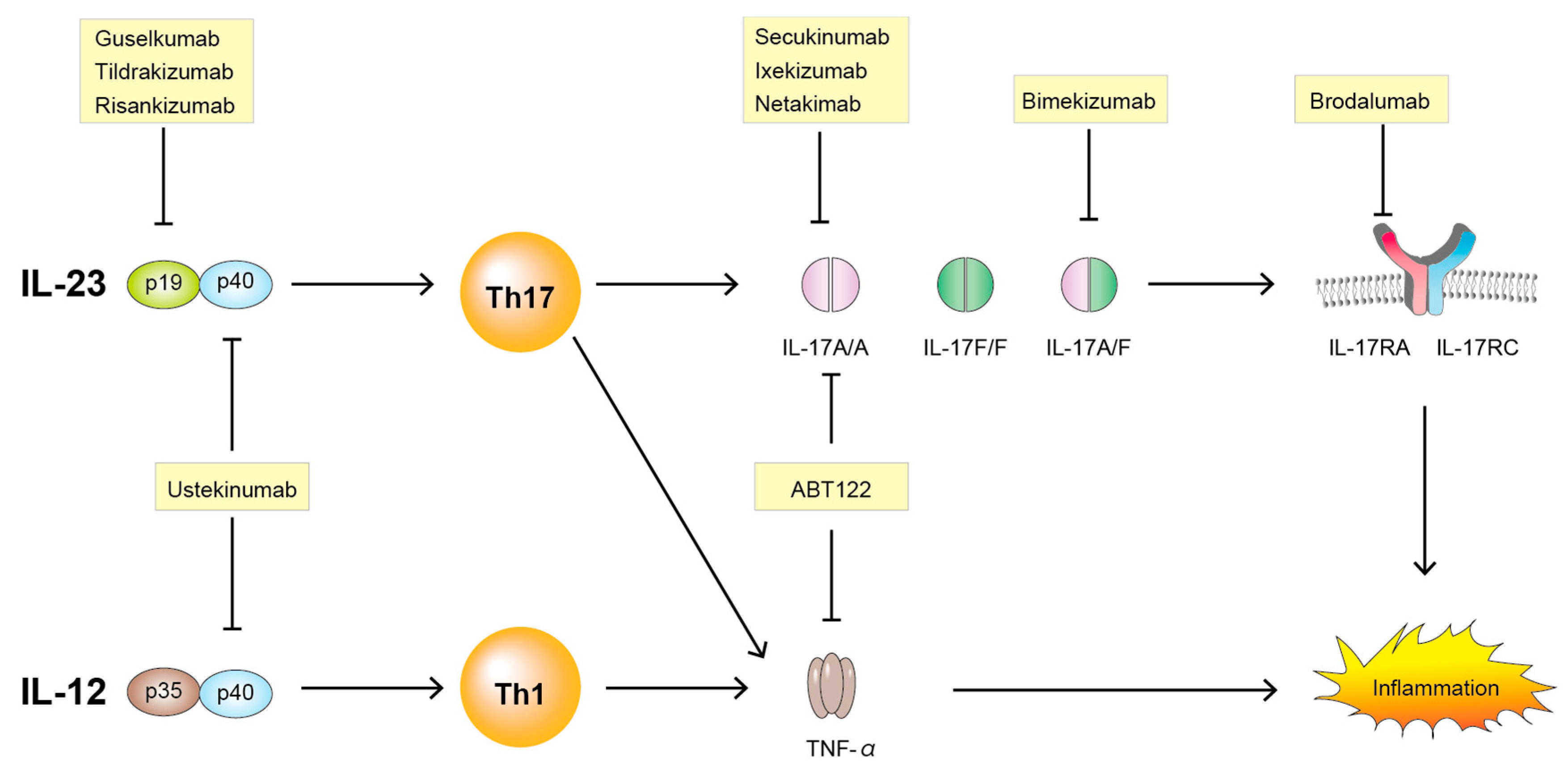 Source: mdpi.com
Source: mdpi.com
Clinical significance of the COSENTYX mechanism of action is unknown. NONCLINICAL TOXICOLOGY Carcinogenesis Mutagenesis. Cosentyx secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A IL-17A cytokine and inhibits its interaction with the IL-17 receptor. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. See Patient Support Options.
 Source: researchgate.net
Source: researchgate.net
Learn More About This Treatment. Ad Get Information About The COSELA Mechanism Of Action. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. Ad Click to View MOA Efficacy Information for WINLEVI clascoterone cream 1. Get Important Safety And Dosing Information.
 Source: cell.com
Source: cell.com
And no increased risk of suicidality has been. On January 21 2015 secukinumab Cosentyx. NONCLINICAL TOXICOLOGY Carcinogenesis Mutagenesis. Cosentyx secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A IL-17A cytokine and inhibits its interaction with the IL-17 receptor. Cosentyxs summary of product characteristics SmPC and its package leaflet give.
 Source: researchgate.net
Source: researchgate.net
The immune pathophysiology of PsA AS nr-axSpA and PsO involves complex molecular mechanisms of. Get Important Safety And Dosing Information. 910 rows Mechanism of action Secukinumab is a human monoclonal antibody that targets IL-17A. See Patient Support Options. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You.

Ad See Full Safety And Prescribing Information Including Boxed Warning. Secukinumab Cosentyx is a human immunoglobulin Ig-G1-κ monoclonal antibody which binds to the IL-17A receptor interrupting the inflammatory cascade 28 and has recently been. And no increased risk of suicidality has been. Watch Videos More. Drugs with different mechanisms of action eg.
 Source: sciencedirect.com
Source: sciencedirect.com
Learn More About This Treatment. Mechanism Of Action. Ask About COSENTYX See If Its Right For You. Learn More About This Treatment. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of.
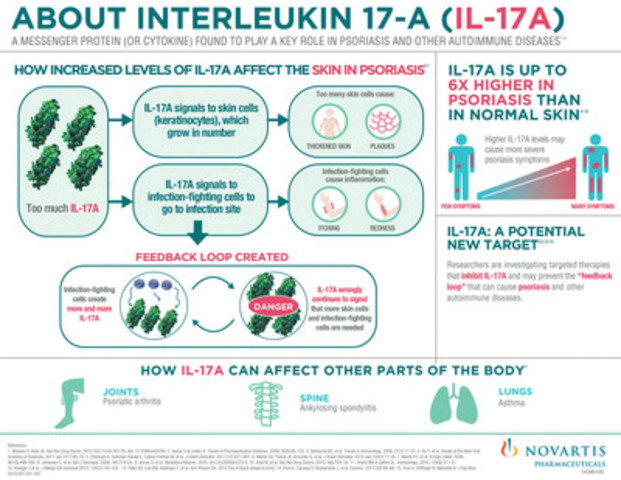 Source: newswire.ca
Source: newswire.ca
NONCLINICAL TOXICOLOGY Carcinogenesis Mutagenesis. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of. Ad XPOVIO is FDA-approved. Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. And no increased risk of suicidality has been.
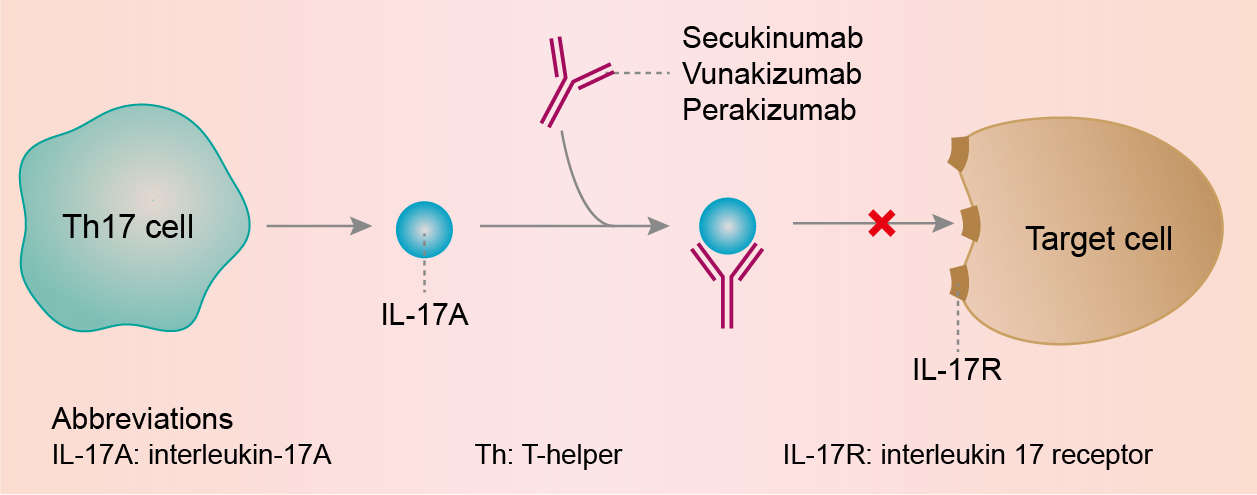 Source: creativebiolabs.net
Source: creativebiolabs.net
Ad Learn How COSENTYX Treats Multiple Conditions See If It Is Right for You. Images not drawn to scale. Clinical significance of the COSENTYX mechanism of action is unknown. Cosentyx secukinumab is used to treat plaque psoriasis ankylosing spondylitis and psoriatic arthritis. COSENTYX is a human interleukin-17A antagonist indicated for the treatment of.

Secukinumab is a human IgG1 monoclonal antibody that selectively binds to interleukin-17A IL-17A and inhibits its interaction with the IL-17 receptor. ZYNLONTA Is An FDA-Approved Treatment Option. Mechanism Of Action. Includes Cosentyx side effects interactions and indications. Visit the Patient site to learn more about it.

Ask About COSENTYX See If Its Right For You. Pay as Little as 25 with the WINLEVI Co-Pay Card. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of. Cosentyx secukinumab is used to treat plaque psoriasis ankylosing spondylitis and psoriatic arthritis. COSENTYX secukinumab.
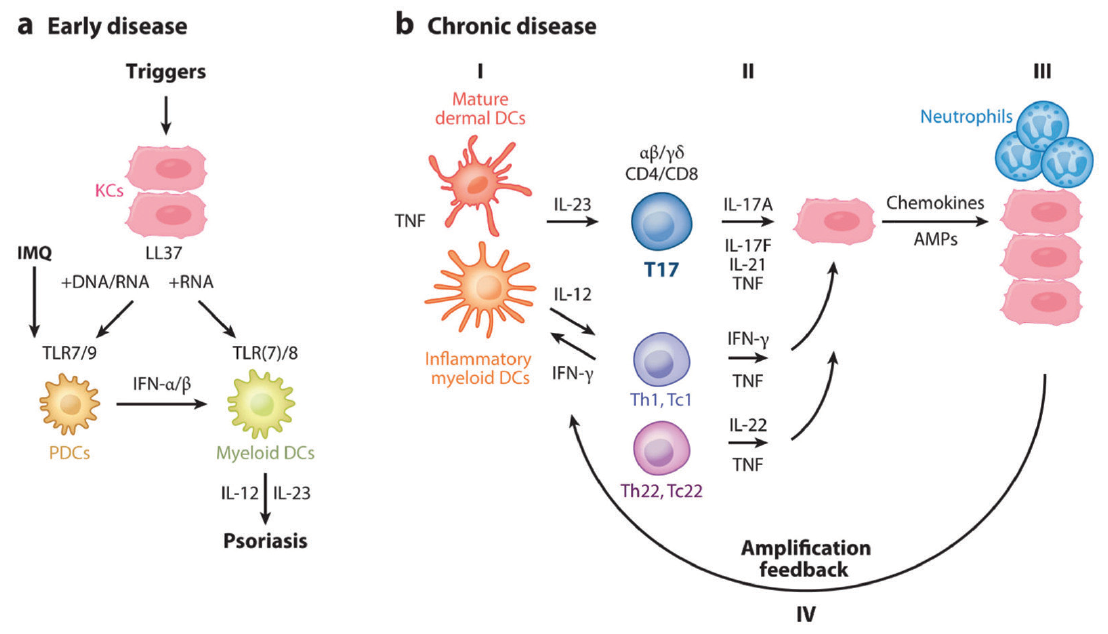 Source: practicaldermatology.com
Source: practicaldermatology.com
On January 21 2015 secukinumab Cosentyx. ZYNLONTA Is An FDA-Approved Treatment Option. Pay as Little as 25 with the WINLEVI Co-Pay Card. 121 Mechanism of Action 122 Pharmacodynamics 123 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 131 Carcinogenesis Mutagenesis Impairment of. Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A IL-17A cytokine and inhibits its interaction with the IL-17.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cosentyx mechanism of action by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






